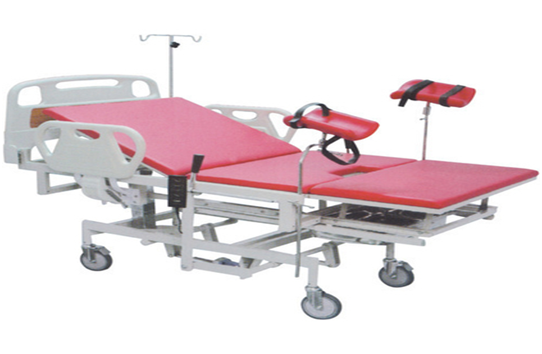
Model No: BMH 77787
- Bed movement functionalityManual control
- Type of delivery bed actuatorMechanical (Handle Operated)
- Type of mechanism for controlling angular motion of bedSuper Smooth Crank Screw Mechanism
- Type of mechanism for fuctioning or controlling height of bedSuper Smooth Crank Screw Mechanism
- Type of side panelCollapsible type
- Number of Side panel2
- Shape of Head panelB-type shape
- Type of Head PanelDetachable type
- Shape of Foot panelB-type shape
- Type of foot PanelDetachable type
- Number of hooks provided in IV Rod2
- Power of motor in HPNA for non motorised
- Facility of instant non powered/mechanical CPR( Cardiopulmonary Resuscitation) release at the head end for emergencyYes
- Urine Bag holder providedYes
- Mattress provided with delivery bedYes
- Mattress foam of the Bed should be with Anti Microbial agent incorporated into all components that assists in Prohibiting growth of bacteria & fungi and easy to clean Yes
- Mattress shall be made of High resilient & bio-density foamYes
- “Power Supply “N/A for Handle operated
- Spares Pair of Bed Ends, detachable supplied1
- Spares Mattress1
- Facility of Resettable overcurrent breaker shall be fitted for protectionYes
- Electric Shock Protection level Class-B/Class -1
- Warranty (Option of comprehensive warranty is available through bidding only, which if opted will supersede normal warranty in the catalogue)3
- Type of powder/Paint coating done to delivery Bed frame,top & side panelN/A for SS
- Process used for pre chemically treatment of metallic bed componentN/A for SS
- Material for the frame of bedSS
- Material for Head & Foot Panel/BoardSS
- Material for Bed top SectionSS
- Material of wheelsSS
- Material for IV RodSS
- Material for waste receptacle bucketSS
- Material for delivery Bed MattressPU Foam
- Length range of delivery bed in mm1851-2000
Special Terms & Conditions
1. Comprehensive warranty:
Comprehensive warranty shall include preventive maintenance including calibration as per technical/ service /operational manual of the manufacturer, service charges and spares,. During the warranty period commencing from date of the successful completion of warranty period, Service personnel shall visit each consignee site as recommended in the manufacturer:��s technical/ service /operational manual, at least once in six months. warranty shall not be including the consumables .Further there will be 98% uptime warranty during warranty period on 24 (hrs) X 7 (days) X 365 (days) basis, with penalty, to extend warranty period by double the downtime period.
2. Service centres:
Details of Service outlets in India to render services for equipment to be furnished to buyer/consignees with complete address ,telephone numbers, e mails etc at time of making the supplies .It shall be the responsibility of seller to ensure that authorized service centres are available to cater to the areas where supplies are made within reasonable distance from where the service calls can be handled .Details of toll free numbers for service call and online registration of service requests also to be provided buyer/consignee at the time of supplies.
3. Source of supply:
It shall be responsibility of seller to provide Documents regarding source of equipments such as copy of Performa invoice or any other documents to establish that the products supplied are manufactured by OEM indicated and sourced from them .
4. Packing and Marking: Medical equipments being very delicate and sensitive packing for the goods should be strong and durable enough to withstand transit including transhipment (if any), rough handling, open storage etc. without any damage, deterioration etc. .The size, weights and volumes of the packing cases, remoteness of the final destination of the goods, availability or otherwise of transport and handling facilities at all points during transit up to final destination,. Quality of packing, the manner of marking within & outside the packages and provision of accompanying documentation shall take in to consideration the type of medical equipments being supplied. The accessories shall be suitably labelled and packed .Each of the package shall be marked on three sides with indelible paint of proper quality: indicating contract number and date , brief description of goods including quantity ,. Packing list reference number , country of origin of goods and any other relevant details.
5. Spare Parts:
Seller shall provide materials, information etc. pertaining to spare parts manufactured and supplied by the OEM . It shall be ensured that the required spares are available for purchase at least for 10 years from date of supplies .In case due to any reasons the production of the spare parts is discontinued sufficient advance notice should be given to the buyer/consignee before such discontinuation to provide adequate time to purchase the required spare parts etc. Further, OEM and their service centres/dealers shall carry sufficient inventories to assure ex-stock supply of consumables and spares for the equipments so that the same are available.
OEM or reseller shall always accord most favoured client status to the buyer/consignee and shall give the most competitive price for spares and consumables of its machines/equipments supplied
6. Installation, Training, Manuals:
Seller shall be responsible to carry out Installation & commissioning, Supervision and Demonstration of the goods. They shall provide required jigs and tools for assembly, minor civil works for the completion of the installation and Training of Consignee:��s representatives for operating and maintaining the equipment and Supplying required number of operation & maintenance manual for the goods. In case the category parameters are specifying any requirements regarding the installations , training and manuals the same shall also be applicable.
7. Electrical safety checking:
Sellers are required to make sure that they furnish the list of equipments for carrying out routine and preventive maintenance to buyer/consignee. They should make sure to periodically check the electrical safety aspects as per BIS Safety Standards or equivalent. In case they do not have required equipment for such testing should ensure that the equipments checked for electrical safety compliance through labs with facilities for such checking during every preventive maintenance call.
8. Software:
All software updates should be provided free of cost during warranty period.
Performance Parameters
| Purpose | It used in Obstetrics/Gynecology deparment for baby delivery in labour room with comfortable requirment of patient and easy accessible professional need for delivery team |
| Bed movement functionality | Manual control |
| Type of delivery bed actuator | Mechanical (Handle Operated) |
| Type of mechanism for controlling angular motion of bed | Super Smooth Crank Screw Mechanism |
| Type of mechanism for fuctioning or controlling height of bed | Super Smooth Crank Screw Mechanism |
| Number of section provided in bed top | 3 |
| Large perineal cut in C shape must be provided in seat section | Yes |
| Side safety belt provided | Yes |
| Type of side panel | Collapsible type |
| Number of Side panel | 2 |
| Head panel/Board provided | Yes |
| Shape of Head panel | B-type shape |
| Type of Head Panel | Detachable type |
| Foot panel provided | Yes |
| Shape of Foot panel | B-type shape |
| Type of foot Panel | Detachable type |
| Provision to convert bed in to table | Yes |
| Good grip provided to head/foot or side panel | Yes |
| Delivery bed should be Hand grip /push grip handles on both side | Yes |
| Adjustable leg rest provided | Yes |
| Lithotomy position & padded knee crutches provided | Yes |
| Slots for IV rod a head section provided | Yes |
| Chromium plated IV rod provided | Yes |
| Number of hooks provided in IV Rod | 2 |
| Power of motor in HP | NA for non motorised |
| Availability of Noiseless, non rusting swivel castor wheel/ roller with for easy Mobility & Steering | Yes |
| Braking and locking mechanism provided in castor roller | Yes |
| No of caster to which braking system provided | 2 |
| Corner buffer provided in all four corner to protect patient from collision shock | Yes |
| Functionality of Foot side panel | Detachable type |
| Safe working load capacity of delivery bed in kg | 150 |
| Facility of instant non powered/mechanical CPR( Cardiopulmonary Resuscitation) release at the head end for emergency | Yes |
| Urine Bag holder provided | Yes |
| Mattress provided with delivery bed | Yes |
| Mattress foam of the Bed should be with Anti Microbial agent incorporated into all components that assists in Prohibiting growth of bacteria & fungi and easy to clean | Yes |
| Mattress shall be made of High resilient & bio-density foam | Yes |
| Operating temperature & Humidity | 10 -40 Degree Celsius and relative humidity of 15-90% |
| “Power Supply “ | N/A for Handle operated |
| Spares Pair of Bed Ends, detachable supplied | 1 |
| Spares Mattress | 1 |
| Facility of Resettable overcurrent breaker shall be fitted for protection | Yes |
| Electric Shock Protection level | Class-B/Class -1 |
| Warranty (Option of comprehensive warranty is available through bidding only, which if opted will supersede normal warranty in the catalogue) | 3 |
| Base frame and support frame should be fabricated using metallic square / rectangular section of adequate cross section and thickness to provide high structural strength and stability | Yes |
| Delivery Bed should be made up of high quality metal, pretreated with materials providing good finish, scratch resistant, bacteriostatic coating | Yes |
| Type of powder/Paint coating done to delivery Bed frame,top & side panel | N/A for SS |
| Process used for pre chemically treatment of metallic bed component | N/A for SS |
| Provision of easily cleaning /sterllize(Especially Blood Stains)/maintenance to delivery Bed | Yes |
| Product instruction manual shall be supplied | Yes |
| Demonstration of equipment and training to be provided after completing supplies before Acceptance | Yes |
Material Parameters
| Material for the frame of bed | SS |
| Material Side Safety belt | Velcro |
| Material for Head & Foot Panel/Board | SS |
| Material for Bed top Section | SS |
| Material of wheels | SS |
| Material for IV Rod | SS |
| Material for bowl | SS |
| Material for waste receptacle bucket | SS |
| Material for delivery Bed Mattress | PU Foam |
Dimensional Parameters
| Maximum Adjistable Back Rest Angle in Degree | 60 |
| Maximum Trendlenburg Angle in Degree | 30 |
| Maximum Reverse Trendlenburg Angle in Degree | 30 |
| Adjustable bed height in mm with 5% tolerance | 550-850 |
| Diameter of Castor Roller in mm | 100 |
| Clearance between Bed Base frame and Floor surface in mm | 125 |
| Maximum thickness of Mattress in mm with tolerance of plus minus 2mm | 65 |
| Approx foam Density in kg/m3 tolerance | 30-50 |
| Therapeutic Weight limit for mattress in kg | 125 |
| Length range of delivery bed in mm | 1851-2000 |
| Width range of delivery bed in mm | 800-900 |
| Weight of delivery Bed in Kg | 80 |
Reports
| Availability of ISO certification for manufacturer | Yes |
| ISO certification No and Date if ISO certificate not available then put NA | |
| Copies of certificates and other certifications to be furnished to buyer on demand at time of supplies | Yes |